Weight-loss drugs. It's a topic that has captivated the masses for the past two years, spearheaded by the miracle-like effects. The rising popularity of glucagon-like peptide 1 (GLP-1) agonists — dominated by Novo Nordisk's Ozempic and Wegovy — have been a thorn in the side for Resmed Inc (ASX: RMD) shares.
At its worst, the Resmed share price plunged 35.5% in eight weeks last year. Since then, shares in the medical device maker have regained part of their former glory. Today, Resmed is trading 35% above last year's trough at $29.30.
Maybe the market is beginning to more closely evaluate how big of an impact weight loss medication will have on demand for devices treating sleep apnea — a sleep disorder commonly experienced alongside obesity.
What better way to stay up to speed with any ramifications for Resmed than by getting direct to the source? If anything is keeping Resmed shareholders up at night, I'd take a guess it would be pharmaceutical giant Novo Nordisk — the figurative boogeyman under Resmed's bed.
Keeping close tabs on a threat to Resmed shares
As a Resmed shareholder myself, I think it's prudent and insightful to read the results of our largest perceived risk. As Sun Tzu put it in The Art of War, "If you know the enemy and know yourself, you need not fear the result of a hundred battles."
Furthermore, any threat is likely to be downplayed within the Resmed community. I'd argue a more true reflection can be obtained by stepping into the dragon's lair.
So, here are three notable takeaways from Novo Nordisk's fourth-quarter 2023 result.
1. Weight loss application is booming
Before obesity care entered the scene, Novo Nordisk was known as a provider of treatments for people with diabetes. That is still true, with the company filling approximately 5 million GLP-1 scripts monthly in the United States for diabetes. However, weight-loss usage (or obesity care) is beginning to rival the company's insulin sales.
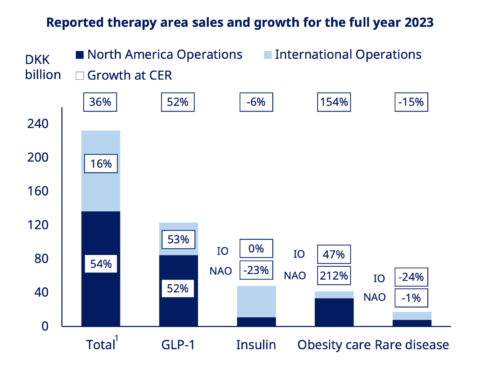
As shown above, obesity care was Novo Nordisk's fastest-growing segment in FY23, with sales surging 154%. This category is sold under the company's Wegovy label, a repackaged formulation of the same active ingredient in Ozempic known as semaglutide.
This is notable for owners of Resmed shares. It demonstrates a strong and growing demand among people affected by obesity. Keep in mind the total estimated addressable population is currently 764 million. So, only a fraction of people are receiving treatment.
2. Beware the tablet
A point against GLP-1s highlighted by Resmed CEO Mick Farrell is the prohibitive price over a lifetime of use — requiring weekly injections. Generally, tablets are a much cheaper vector for delivery and come with fewer complications.
Novo Nordisk already makes Rybelsus, the first oral GLP-1 agonist, except its small dosage is for treating type-2 diabetes. However, the company is showing promising results for a 50-milligram version for the obesity care demographic.
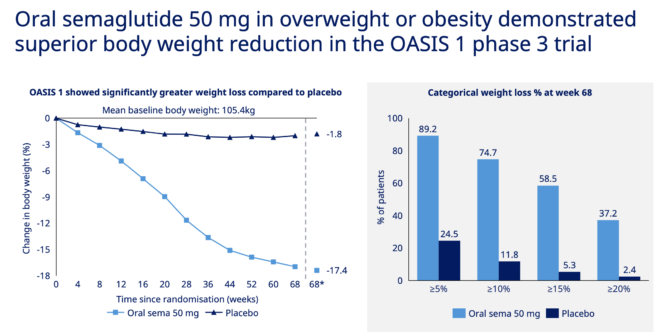
Trials have yielded a weight loss of 17.4% after 68 weeks compared to a 1.8% reduction baseline, as shown above. Slated to be a once-a-day tablet for weight loss, Novo Nordisk is currently undertaking phase 3 trials.
A tablet could unlock a much bigger market for GLP-1s, making it more tolerable and cheaper. If a strong link is made to reduced sleep apnea cases, this could present a risk to Resmed shares.
3. Misuse an unlikely target
Rumours of wide use by celebrities and the broader public as a 'get-slim-quick' drug might well be overdone.
During the conference call, Nadim Rizk enquired about the percentage of 'casual users' and whether it could spark a crackdown by the Food and Drug Administration (FDA). Novo Nordisk executive VP of commercial strategy and corporate affairs, Camilla Sylvest, responded:
On the data that we have, especially from our launch in the U.S., we see from our real-world evidence that the average BMI of the people who are treated with Wegovy is approximately around a BMI of 37. This has also been confirmed in additional countries where we have launched in Europe. So that is sort of the average BMI, well above the label that we have that is BMI of 27 with comorbidities or above 30.
Averages are not always representative. However, it does suggest the medication is being prescribed appropriately.









